Unless you work in biopharmaceutical research and development (R&D), chances are the process of developing new medicines seems like a black box. It is hard to imagine how researchers go from an initial idea, based on the underlying biology of a disease, to the development of a safe and effective treatment, and the many complex challenges researchers face in getting there.
PhRMA has released a new resource, “Biopharmaceutical Research & Development: The Process Behind New Medicines,” which explains the complexities inherent in developing a new medicine for patients. Our increasing understanding of the underlying biology of many diseases creates tremendous opportunity for the development of innovative new medicines. In order to deliver on the promise of the science, biopharmaceutical companies are working hard to develop innovative approaches and advance regulatory science to address technical, scientific, and regulatory challenges.
Here are five things you may not have realized about this intricate process:
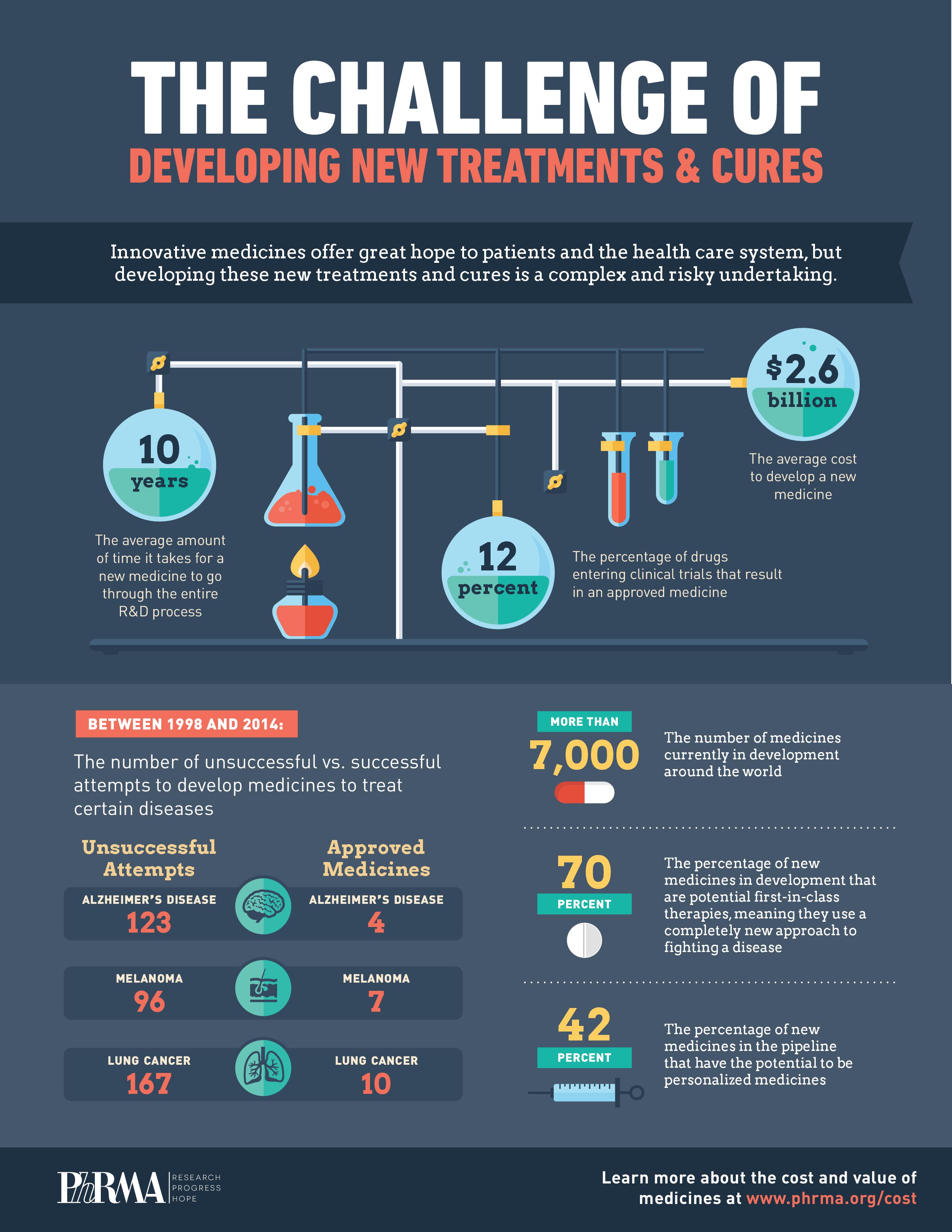
- Less than 12% of the medicines that enter clinical testing will be approved by the FDA. More often than not, the R&D process ends in failure. Researchers learn from these setbacks and use them to inform future research.
- On average, it takes at least 10 years for a new medicine to complete the journey from initial discovery to FDA approval.
- The way clinical trials are conducted is constantly evolving. Science is moving very quickly and biopharmaceutical companies continually adapt in many ways, including advancing innovative clinical trial designs and methodologies, enhancing appropriate patient engagement in the development process, and increasing the use of modern drug development tools.
- Biopharmaceutical companies are working with multiple stakeholders including FDA, patient advocacy groups, and academic and government researchers to advance regulatory science and identify new approaches to developing new medicines. These partnerships and collaborations, across the research ecosystem, are crucial in order to deliver new medicines to patients.
- Patient volunteers are one of the most important elements of clinical trials. Stakeholders work together to ensure that patient safety is protected. Recruiting patients for these studies can be very difficult.
Researching and developing new medicines is a long, complex process but biopharmaceutical companies are committed to advancing the science in order to deliver innovative medicines to patients.
Watch the new video to help you learn more about why developing a new drug is harder than rocket science.
{{cta('38e52880-c743-48dc-a663-8761f8dc2567')}}






