Today, as we mark World AIDS Day and commemorate those who lost their lives to AIDS-related illnesses, we want to recognize the impressive advancements in HIV/AIDS scientific research and drug development that have taken place over the past four decades. These innovations in disease prevention, management and treatment have led to dramatic declines in death rates, allowing those living with HIV/AIDS to enjoy longer, fuller lives.
According to the U.S. Centers for Disease Control and Prevention (CDC), HIV, or human immunodeficiency virus, is a virus that attacks CD4 cells (T cells), which are an important line of defense in a person’s immune system. If HIV is left untreated, it can lead to AIDS (acquired immunodeficiency syndrome), which means that a person’s CD4 cells fall below a certain threshold and are no longer able to return to their normal level. Today, nearly 1.2 million people are living with HIV in the United States.
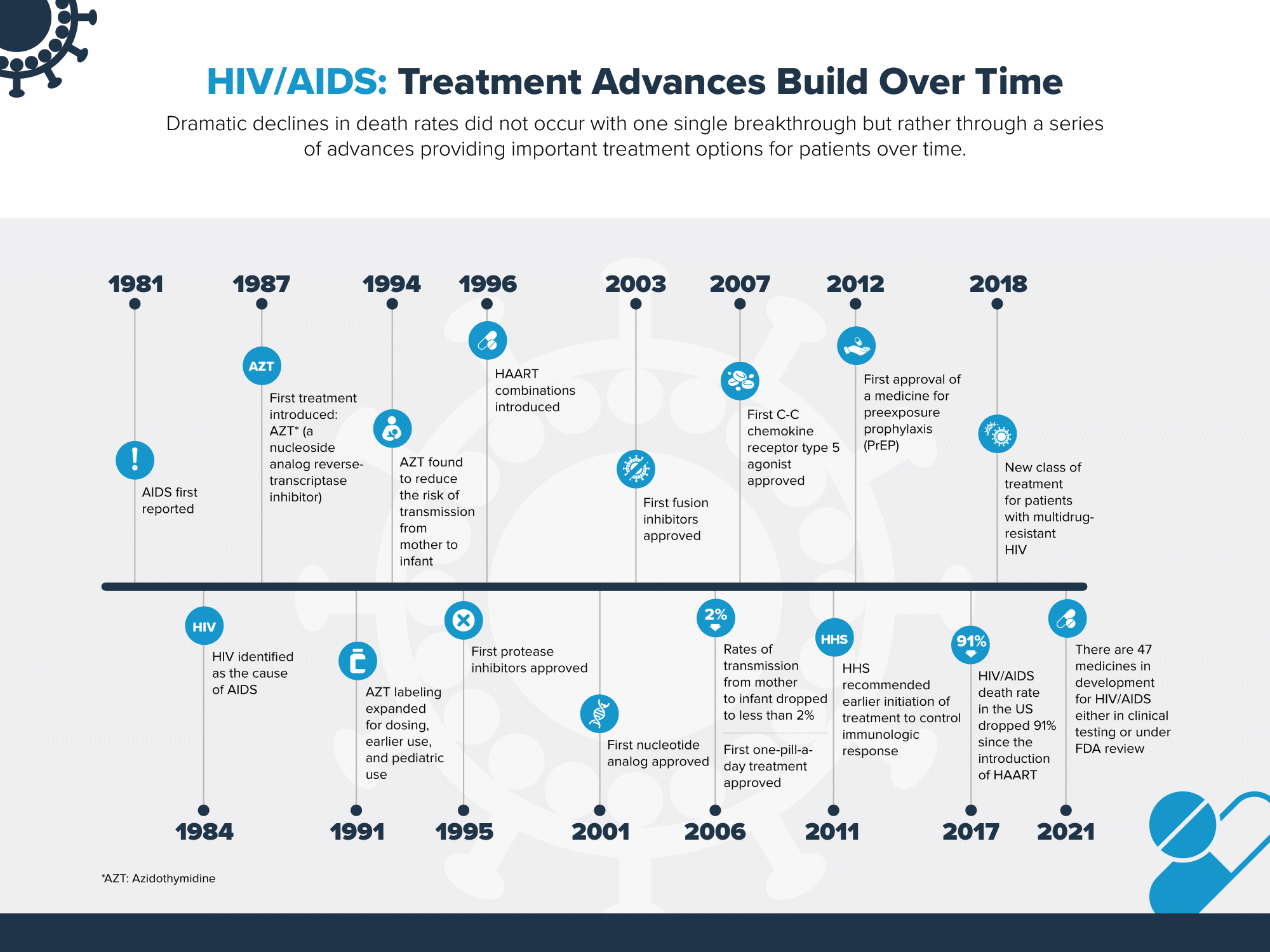
However, since the discovery of the virus, HIV/AIDS research has transformed treatment for patients, leading to better health outcomes for those affected by the disease and reducing spread of the disease overall. In fact, today patients can expect to live a normal life span with appropriate treatment.
As we look back on HIV/AIDS research, the progress has been challenging but also inspiring. In 1987, scientists reached the first medical milestone with the discovery that antiviral nucleoside reverse transcriptase inhibitors (NRTIs) slowed the progression of AIDS in advanced cases. In 1995, biopharmaceutical researchers reached another breakthrough: the discovery of antiviral protease inhibitors, which halted the spread of the disease by preventing infected cells from duplicating and spreading the HIV virus. These new treatments marked the beginning of highly active antiretroviral therapy (HAART), which quickly revolutionized the fight against HIV/AIDS.
The biopharmaceutical industry has worked rigorously to continue improving medicines for treating people with HIV/AIDS by developing antiretroviral (ART) treatments with fewer side effects, combinations that improve adherence and pre-exposure prophylaxis medicines (PrEP) to help prevent transmission of HIV.
As scientists learned more about HIV through years of research, treatment regimens have become simpler and eliminated certain adverse side effects. In particular, the development of combination therapies reduced many people’s treatment regimens from multiple pills per day to a single, once-a-day combination medicine. Collectively, these advancements not only made it easier for people to take their medicines and adhere to their treatment plans, but also allowed people with HIV to have longer life expectancy.
While the HIV/AIDS epidemic has affected millions of lives, it also played a role in how the U.S. Food and Drug Administration (FDA) reviews medicines. Importantly, near the beginning of the epidemic, HIV/AIDS activists brought their concerns about a lack of available treatment options for the virus directly to the steps of the U.S. government and worked to raise awareness around key clinical trials at a time when death rates were increasing. AIDS activist group ACT UP! held demonstrations across the United States, including closing down the FDA for a day in 1988, to protest the slow process of drug approval driven in part by a lack of FDA resources. These activities have had far reaching impact on drug development overall, spurring FDA, Congress and industry to work together to help ensure FDA is resourced appropriately to support the efficient and timely regulatory review process for innovative medicines through the creation of the Prescription Drug User Fee Act (PDUFA) established in 1992.
Today, the HIV/AIDS death rate in the United States has dropped 91% since the introduction of HAART and people are living longer, healthier lives. However, research and medical innovation cannot stop here. HIV continues to disproportionately impact certain populations, particularly racial and ethnic minorities and gay, bisexual, and other men who have sex with men. In 2019 alone, an estimated 34,800 new HIV infections occurred in the United States.
Though progress in HIV/AIDS research has made tremendous strides over the past several years, the biopharmaceutical industry remains dedicated to working in collaboration across the ecosystem towards continued medical innovation and on day, a cure. As of 2021, there are nearly 50 medicines, including vaccines, in development for HIV/AIDS either in FDA review or in clinical trials.
To learn more about HIV/AIDS innovation, read the full fact sheet here.
This blog post is dedicated to all the people who have been impacted by HIV/AIDS. We especially want to recognize one notable activist over many years, William Arnold, who recently passed away. According to news reports, Mr. Arnold “was a founding director and served since 1996 as president and CEO of the Community Access National Network (CANN), which advocates for affordable health care services and support for people with HIV/AIDS and viral hepatitis.”






