Today, PhRMA released a new report covering 549 medicines in development to potentially treat disorders of the blood, including blood cancers. These conditions pose a significant and ongoing unmet medical need in the United States. To meet the demand for new treatments, companies in America’s biopharmaceutical industry have spent years working across the biomedical ecosystem to research and develop new medicines that employ the latest in scientific and technical knowledge.
Blood helps the human body perform functions vital to staying alive. Red blood cells deliver oxygen to the body, white blood cells help fight against infection, platelets (colorless blood cells) help blood clot and plasma helps to deliver nutrients and remove waste products. Disorders of the blood cover a broad range of diseases that come with different origins, symptoms and treatments. For example, blood disorders that affect clotting function and platelets can lead to hemophilia, blood clots and anemia. While disorders that affect white blood cells may lead to blood cancers, such as leukemia, lymphoma and myeloma.
Managing blood and bleeding disorders creates a tremendous burden for patients as they often require lifelong treatment to manage their condition and prevent serious complications.
Disease management can include routine drug infusions or blood transfusions in addition to frequent visits to physician offices, infusion centers or even the emergency room. Patients with these disorders often experience debilitating pain, disability, reduced life expectancy and lower quality of life.
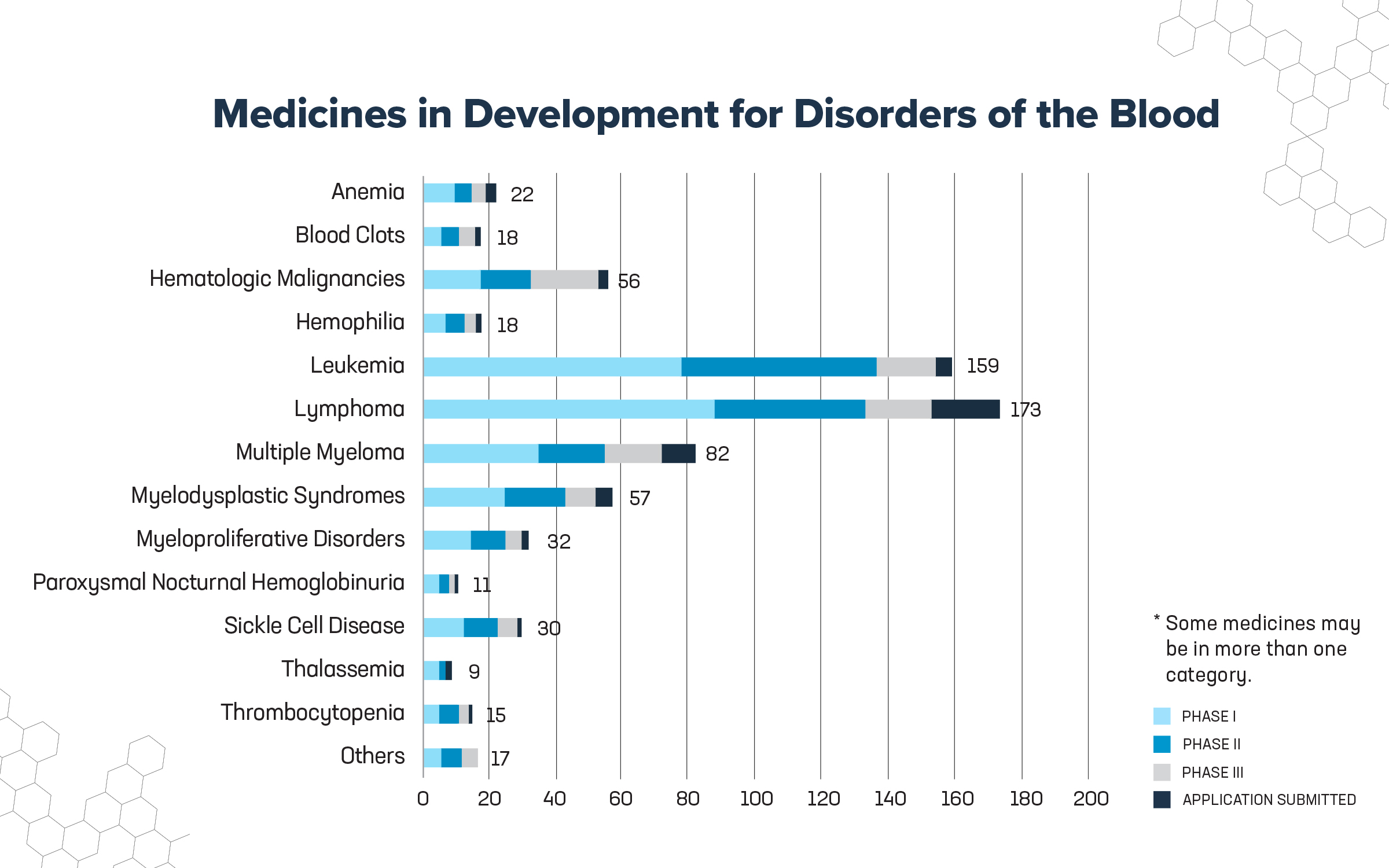
Fortunately, new advancements in science and technology are being employed by the biopharmaceutical industry to develop 549 medicines targeting disorders of the blood. These medicines are either in clinical trials or under review by the U.S. Food and Drug Administration (FDA) and include:
- 162 for lymphoma, which account for nearly 5% of all new cancer diagnoses. Non-Hodgkin lymphoma accounts for 90% of all lymphomas.
- 158 for several types of leukemia, which account for more than 3% of all new cases of cancer. The most common types of leukemia in American adults are chronic lymphocytic leukemia (38%) and acute myeloid leukemia (31%). In children and adolescents, 75% of leukemia cases are acute lymphoblastic leukemia.
- 84 for multiple myeloma, with an estimated 34,470 new cases and 12,640 deaths expected in 2022.
- 73 for hematologic malignancies, cancer that is caused by the uncontrolled division of abnormal cells and can lead to leukemia, lymphoma and multiple myeloma.
- 55 for myelodysplastic syndromes, a group of conditions that happen when the blood-forming cells in the bone marrow become abnormal leading to a low number of blood cells.
- 30 for myeloproliferative disorders, such myelofibrosis, essential thrombocytopenia and polycythemia vera.
- 30 for sickle cell disease, a red blood cell disorder that causes normal round, flexible cells to form into a crescent or sickle shape. These malformed blood cells clog blood vessels preventing normal flow of nutrition and oxygen throughout the body, causing pain, organ damage and a low blood cell count. Sickle cell affects about 100,000 Americans.
- 23 for various forms of anemia, which affect more than 3 million Americans and collectively are the most common bleeding disorder in the United States.1, 2
- 19 for platelet disorders, including thrombocytopenia, a disease characterized by an abnormally low level of platelets in the blood.
- 18 for hemophilia, a genetic disorder classified as type A when caused by a factor VIII deficiency, while type B is caused by a factor IX deficiency (there are other less common types). Between 30,000-33,000 people in the U.S. have a form of hemophilia.
- 14 for clotting disorders, consisting principally of deep vein thrombosis, where a blood clot is located in the deep veins or the arms or legs, and pulmonary embolism, where a clot has traveled from a deep vein to the lung. About 100,000 Americans die each year from blood clots.
- 13 for paroxysmal nocturnal hemoglobinuria, a rare disease of the blood characterized by the destruction of red blood cells, blood clots and impaired bone marrow function.
- 9 for thalassemia, an inherited blood disorder where the blood doesn’t make enough hemoglobin leading to red blood cells that don’t function correctly and only last a short amount of time.
- 16 for other blood disorders, such as erythropoietic protoporphyria (an inherited condition that can cause pain when patients are exposed to light), hemorrhage and retinal vein occlusion (blood clots in the eye veins), among others.
These new treatments employ the latest in scientific and medical knowledge, with many of the 549 medicines representing new and innovative ways to target disorders of the blood, one of the most notable being gene therapies. Potential gene therapies may offer long-term benefits and even cures for blood disorders, some with just a single course of treatment. For example, gene therapies in the late stages of development have been able to significantly reduce bleeding rates and almost entirely eliminate the need for factor replacement therapy for Hemophilia A and Hemophilia B patients in the years following a one-time administration.
The potentially transformative impact of gene therapies may dramatically improve the quality of life for patients while drastically reducing the burden and costs often associated with the current standard of treatment. These savings are significant within a year following administration of a gene therapy but may also accrue over the lifetime of a patient.
The medicines in development today represent the continued commitment of biopharmaceutical companies to build on the learnings from fundamental biology and overcome the challenges inherent in translating these learnings into medicines for disorders of the blood and meet the unmet needs for patients and their families. Looking ahead, despite significant breakthroughs in the treatment for disorders of the blood, incentives to encourage and foster biopharmaceutical innovation and advance current knowledge are still needed to ensure patients have access to these novel treatments.
To read more on the medicines in development for disorders of the blood, click here.
[1] National Heart, Lung and Blood Institute, National Institutes of Health
[2] American Society of Hematology






