Recognizing Hepatitis R&D on World Hepatitis Day
World Hepatitis Day is a chance to celebrate medical advancements that have been life-changing for patients.

Recognizing Hepatitis R&D on World Hepatitis Day
World Hepatitis Day is a chance to celebrate medical advancements that have been life-changing for patients.

Recognizing Hepatitis R&D on World Hepatitis Day
Hepatitis is an inflammation of the liver, most commonly caused by a viral infection. There are five main hepatitis viruses, with types A, B, C most common. In 2018, the Centers for Disease Control and Prevention estimated that Americans were infected with 24,900 cases of hepatitis A and 21,600 cases of hepatitis B, many of which go undiagnosed because individuals do not know they are infected. Over the past several years, the biopharmaceutical industry has made significant progress in bringing medicines to patients that improve cure rates, reduce side effects and duration of treatment and expand treatment options.
In recognition of World Hepatitis Day, we are taking a closer look at the progress that has been made in treating and preventing forms of hepatitis.
Hepatitis A and B are vaccine preventable
Vaccines play a critical role in bolstering immunity and fighting disease in people of all ages, and fortunately, there are vaccines to prevent the spread of both hepatitis A and hepatitis B. Before leaving the hospital, newborn infants often receive their first dose of the hepatitis B vaccine, and a year later, they receive the hepatitis A vaccine. When a high concentration of vaccinations, like those for hepatitis A and hepatitis B, is attained within a community with an effective vaccine, disease transmission can be successfully disrupted. When disease transmission is disrupted, even those who were not vaccinated, or those who did not receive immunity from the vaccine, benefit from vaccination and can be protected from the disease.
Hepatitis C (HCV) is treatable with antivirals
Hepatitis C, or HCV, is a devastating viral disease that progresses slowly over time and can cause very serious damage to the liver. Today, around 2.4 million Americans have Hepatitis C and roughly 50% do not know they are infected with the virus. Sadly, the disease is the leading cause of liver cancer and the most common reason for liver transplant. However, recent scientific advancements are transforming treatment of HCV and have led to a new era of treatments, with cure rates approaching 100% for patients with all known genotypes of the virus.
Direct-acting antivirals (DAA) are oral medicines taken over the course of 8-12 weeks and target HCV at each step of the virus’ lifecycle. Currently, several DAAs are approved for use in patients with all known forms of disease (including difficult to treat subtypes) in the United States. This stands in stark contrast to the medicines available just a decade ago, which were injected over 48 weeks, offered cure rates of just 41% and often caused severe flu-like side effects. Researchers have predicted that with the current public health preventive measures and available treatments, including DAAs, HCV can become a rare disease by 2036.
These developments, especially the recent progress for treatment of HCV, have had dramatic benefits for patients. In fact, there has been a 167% increase in worker productivity for patients with HCV due to new innovative and successful treatments with fewer side effects. More broadly, the advancements have resulted in health care system savings that are much greater than originally predicted.
Additionally, in less than a year after market entry of the first breakthrough treatment for hepatitis C, multiple other products entered the market that offered improved cure rates for patients. The resulting competition was so fierce that the average net daily cost for this class today is nearly 80% lower than the first product’s launch price.
Further, by 2028, the predicted annual cost savings to Medicaid from curing HCV are projected to be 10 times greater than treatment costs, cumulative savings will surpass $52 billion.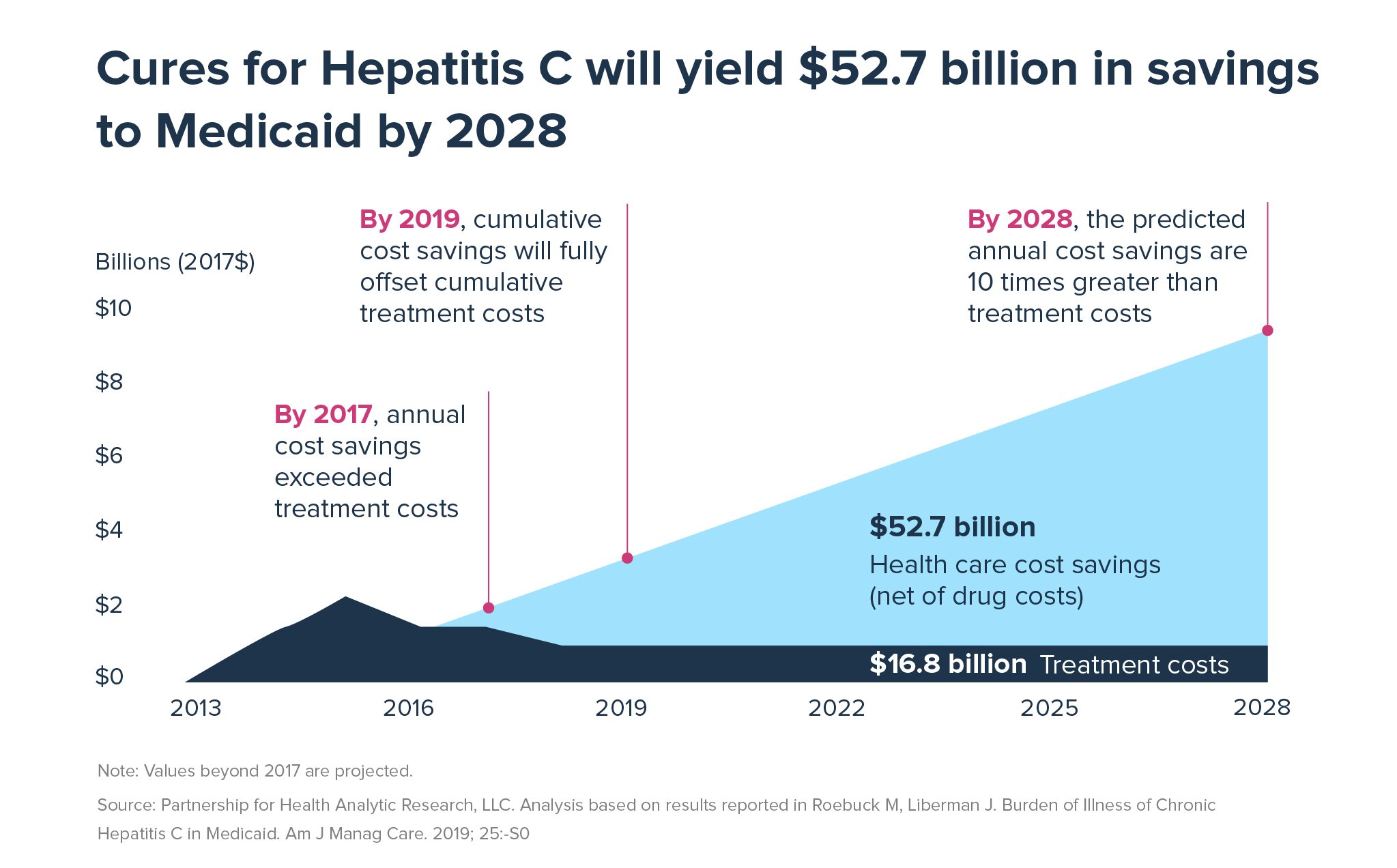
World Hepatitis Day is a chance to celebrate medical advancements that have been life-changing for patients. It is also an opportunity to recognize the research and development that has helped fuel this progress. Milestones including the availability of effective vaccines for hepatitis A and B and a treatment for hepatitis C prove the elimination of viral hepatitis is achievable.
The pipeline for new treatments for forms of hepatitis is strong with nearly 60 medicines in development. In order to continue making progress, greater awareness and understanding of the disease and associated risks is a must. While biopharmaceutical companies continue to advance additional medicines for hepatitis, policymakers should also take a holistic approach to lowering patient costs while supporting medical innovation and ensuring Americans have access to new treatments. This environment is critical to support the continued innovation that patients with hepatitis so desperately need.
1. PhRMA Analysis of AdisInisght Database. June 2021