America’s biopharmaceutical researchers are committed to studying, developing and testing medicines to meet the unique needs of pediatric patients. New treatment options for infants, children and adolescents can be complex and often require different clinical approaches than adult treatment pathways. According to a new report released today, there are nearly 600 pediatric medicines currently in development.
Here is a closer look at a few exciting developments in pediatric medicines:
There are more than 2,100 industry-sponsored pediatric clinical trials underway, testing 580 investigational medicines and involving more than 1.2 million pediatric patients across a variety of therapeutic areas, including diseases where there is significant unmet medical need. Medicines in development include:
- A gene-edited cell therapy that could potentially be a one-time treatment for sickle cell disease
- A monoclonal antibody approved to treat asthma in adults and children ages 12 years and older being tested in children ages 6 to 11
- The first DDP-4 inhibitor approved for adults with type 2 diabetes in the United States being tested in children ages 10 to 17
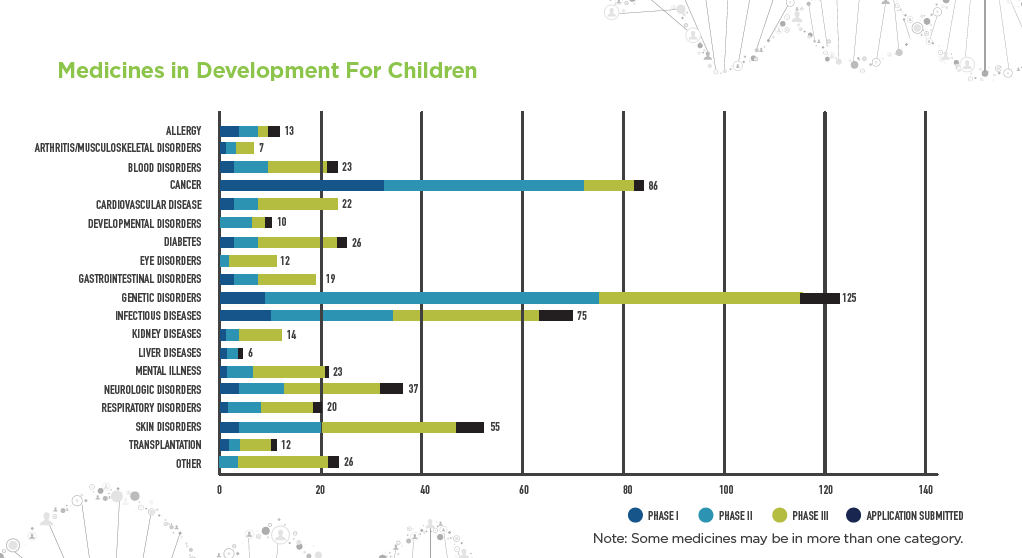
Researchers are also testing medicines approved for use in adults to determine safe and effective dosage levels for children. Current investigations include:
- 125 treatments for genetic diseases including medicines for cystic fibrosis, which affects more than 30,000 American children and adults
- 86 treatments for cancer which, despite significant progress, is still a leading cause of death by disease among American children ages 1 to 19
- 75 medicines for infectious diseases, such as HIV/AIDS, ear infections, pneumonia and hepatitis
- 55 medicines for skin disorders, including atopic dermatitis, a chronic condition which affects about 20% of children in the United States
The Best Pharmaceuticals for Children Act (BPCA) and Pediatric Research Equity Act (PREA) work together to foster pediatric drug development. These acts were permanently reauthorized in 2012, and create a balanced approach that generates important safety and efficacy information on use of medicines in children and enables biopharmaceutical companies to continue to make significant investments in pediatric drug research.
PREA and BPCA are widely regarded as a success for patients, driving significant increases in pediatric research, product approvals and approved labeling for the pediatric population. Before BPCA and PREA became law, more than 80% of the medicines approved for adult use were being used in children, even though the safety and effectiveness had not been established in children. By 2012, that number had been reduced to about 50%. Continued commitment from the biopharmaceutical industry has resulted in great strides against pediatric diseases, including HIV/AIDS, asthma, rare diseases and many forms of pediatric cancer (particularly blood cancers).
While America’s biopharmaceutical researchers have made significant progress to overcome the unique scientific challenges associated with pediatric drug development, more work remains to develop innovative treatment options for pediatric patients.
Learn more at https://www.phrma.org/en/Pediatrics.






