America’s biopharmaceutical companies are at the heart of a research and development (R&D) ecosystem that develops more innovative medicines than any other country in the world. Critical to this ecosystem is the working relationship between industry and government agencies like the National Institutes of Health (NIH), academic medical centers and community-based research sites to further the translation of basic research into important medical products.
A new report analyzed NIH research grants awarded in fiscal year 2000, identifying those associated with clinical trials and medicines approved by 2020, and quantifying the public and private investments made for each. The report’s findings demonstrate that even in cases where NIH research is linked to patented discoveries, ongoing development of new, lifesaving and life-improving medicines would not be possible without the robust investment and shouldering of investment risk, scientific expertise and drug development and manufacturing capabilities of biopharmaceutical research companies.
Key findings from the report include:
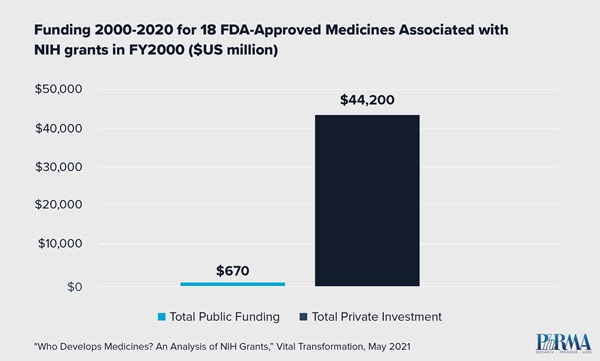
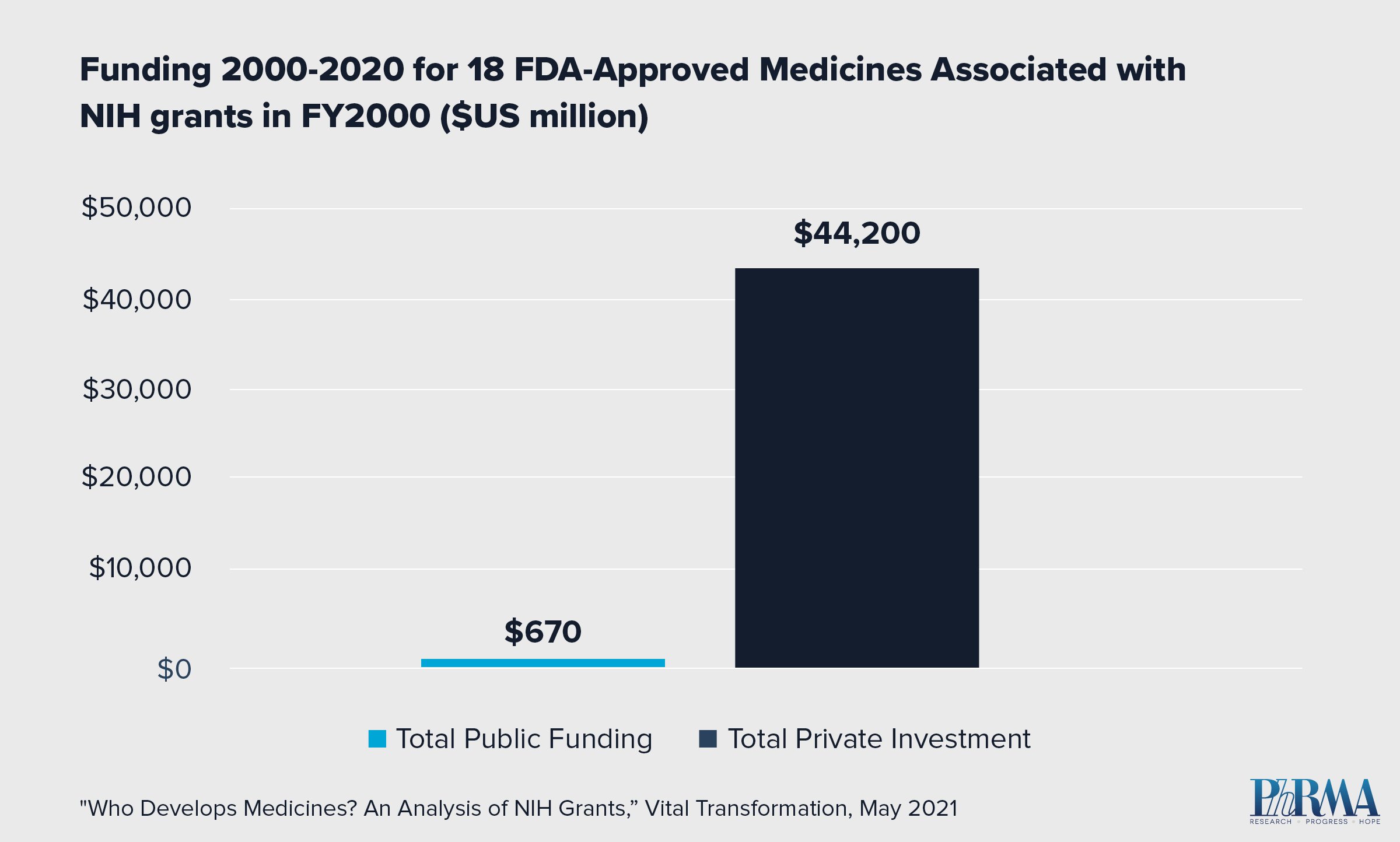
- 23,230 NIH grants issued in the year 2000 were linked to patents associated with 18 FDA-approved medicines by 2020.
- Of those 18 medicines, none reached FDA approval without significant private investment to further the drug development process.
- In fact, private sector investment for the 18 approved medicines exceeded NIH funding by orders of magnitude. Private sector investment totaled $44.2 billion compared to $670 million in NIH funding.
Note also that medicines associated with NIH-supported patents, which are the focus of this study, are a small share of all FDA-approved medicines. A 2019 analysis of nearly 200 top-selling retail medicines found that only 10% listed government-supported patents among the key patents protecting the product.
All of this is consistent with what we know about the overall levels of drug-related R&D spending by public and private sources. For example, in 2018, the biopharmaceutical industry invested $102 billion in R&D, 100% of which was focused on drug development. Meanwhile, the entire NIH budget in 2018 was $35.4 billion, only 8% of which was focused directly on research related to drug development.
The biopharmaceutical industry’s unique role in the research ecosystem is to utilize its scientific and industrial expertise to take the necessary risks to build on and translate basic science research into safe and effective treatments that can be made available to patients. The federal government simply does not research, develop and manufacture vaccines and other new treatments on its own. Rather, publicly funded research catalyzes private sector investment, bringing to bear the resources, scientific expertise, R&D, manufacturing and technological platforms of biopharmaceutical companies to bring medicines to market. A recent Congressional Budget Office report summed it up when they found that public-sector research and private R&D “are complements, not substitutes.”
Proponents of policy proposals like those in the House bill known as H.R. 3, which would markedly reduce the private sector role in biopharmaceutical R&D, argue that increasing public research funding could make up for that loss. This study adds to the large body of evidence showing that industry investment is essential to translate scientific discoveries into treatments. Policies like those in H.R. 3 would undermine this system and jeopardize biopharmaceutical innovation across the board, as has happened in countries with similar policies. For example, most innovative medicines used to be developed in Europe. However, as European countries began adopting policies like H.R. 3, that leadership slipped away.
Instead, policymakers should pursue policies that support medical innovation and ensure Americans have access to the best treatments available. Learn more here: PhRMA.org/BetterWay