One of the biggest myths regarding the administration’s proposed International Pricing Index (IPI) Model is that it will have little to no effect on biomedical research and development (R&D). Secretary of Health and Human Services Alex Azar has said that the reduction in R&D couldn’t possibly be more than 1 percent. In contrast, PhRMA estimates that the impact of the model on R&D is much greater with a disproportionately negative effect on innovative medicines, including new medicines that are meeting unmet medical needs for previously untreatable conditions, like some cancers and potential cures for genetic disorders.
In December, PhRMA commissioned the law firm of Manatt, Phelps & Phillips, LLP to survey PhRMA member companies to see how they expect the proposal to affect their R&D. Here are some of the key findings from survey respondents:
- 77 percent of companies said that if the proposal goes through, it will affect their ability to pursue current or future R&D projects.
- 92 percent of companies saw a risk of reducing investments in all Part B medicines, which are primarily complicated biologics that are administered by physicians and used to treat multiple sclerosis, rheumatoid arthritis, cancer and genetic disorders.
- 70 percent of companies expected significant cuts in cancer R&D.
- Half of the companies reported that 20 percent or more of their current projects could be reduced or terminated.
The survey also reminds us that industry R&D is about even more than finding new treatments and cures. Companies also reported potential economic impacts: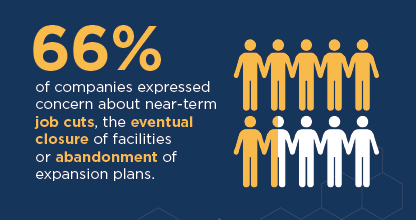
- 66 percent of companies expressed concerns about near-term job cuts, the eventual closure of facilities, or the abandonment of expansion plans.
- 80 percent of companies said the proposal would reduce their opportunities to partner with early stage biotech companies. Small biotech companies often partner with larger companies for support throughout the R&D process.
Unprompted, several companies said that the proposal would actually reduce competition in certain classes by stifling the development of biosimilars. Recent studies project that biosimilars could reduce spending on biologics by between $25 to $150 billion over the next 10 years. However, policies that would drastically reduce payment for these types of medicines could undermine incentives for development, deterring a marketplace that has not yet reached maturity.
The R&D impact of the IPI proposal is far beyond “1 percent.” New and current projects focused on treating diseases like cancer are at risk, as are jobs and the future of small innovative companies. The administration should abandon this proposal and pursue market-based solutions that focus on improving affordability and care for patients in Medicare Part B.