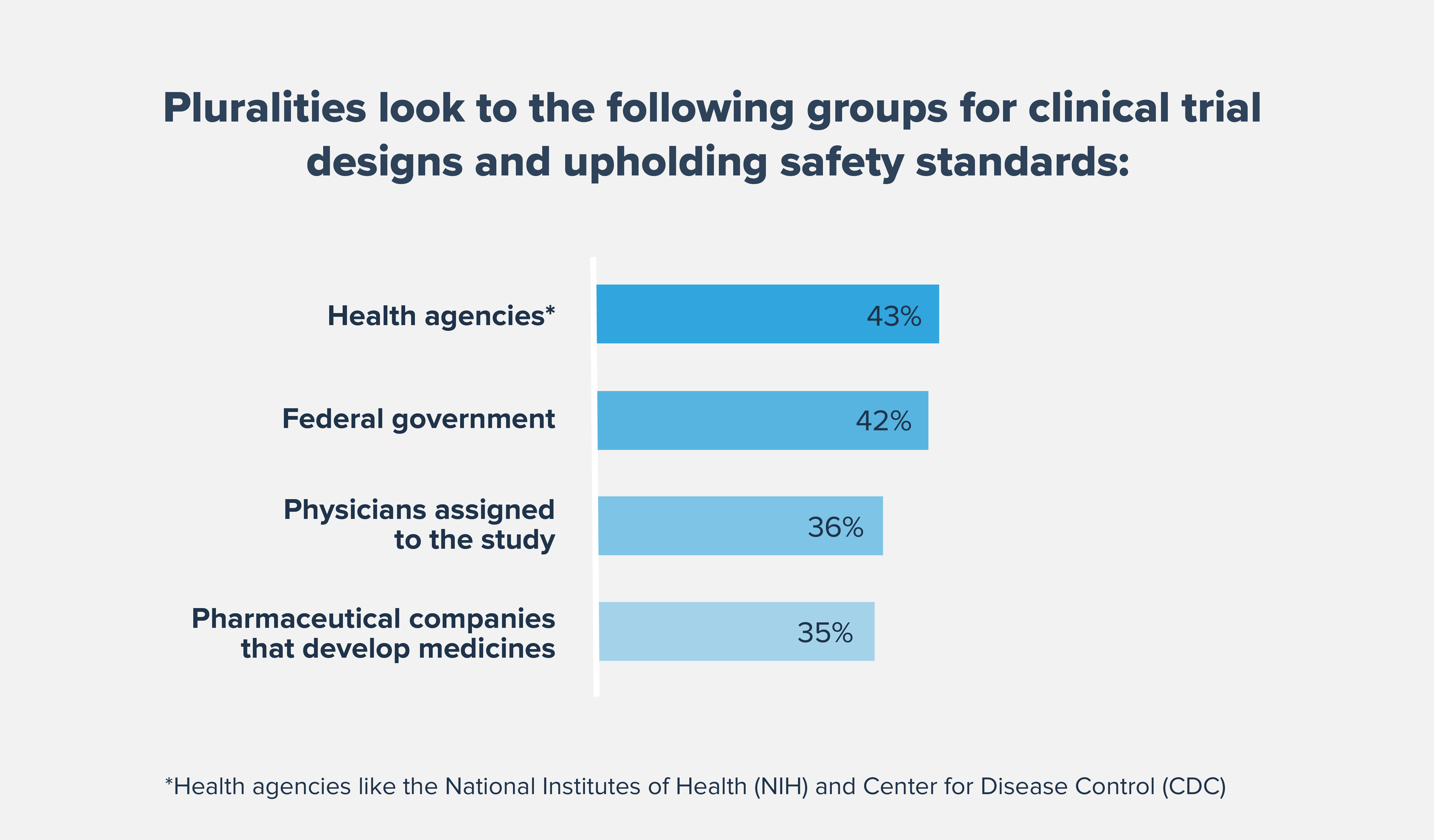
Improving Clinical Trial Diversity is Critical to Health Equity
Enhancing clinical trial diversity is a highly complex challenge that requires a community-based, multi-stakeholder approach.
Learn more about PhRMA’s efforts to address the systemic barriers that can deter underserved communities from participating in clinical trials, so that people who want to participate, can.




Knocking Down Systemic Barriers and Changing the Paradigm to Enhance Clinical Trial Diversity
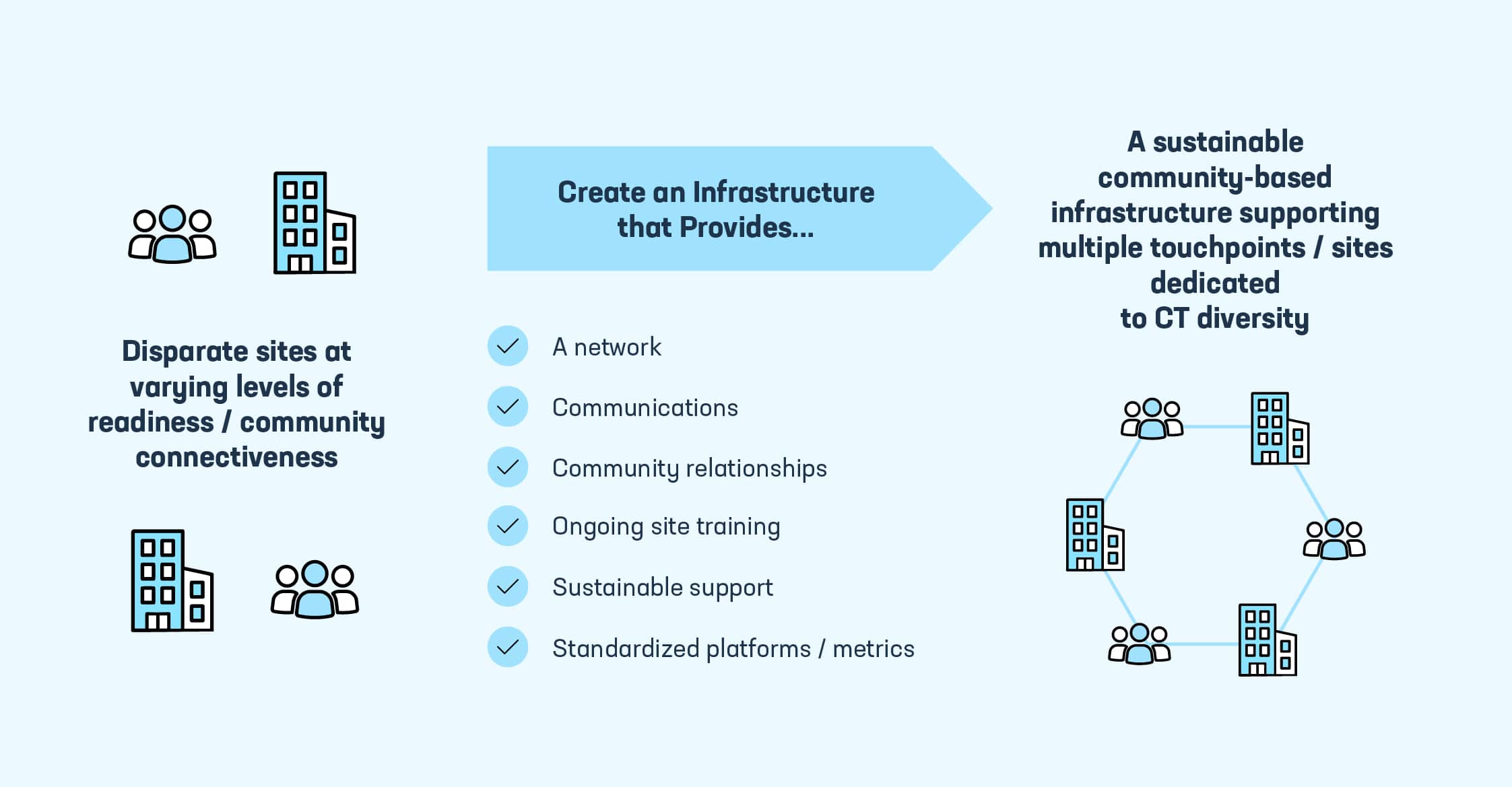
Since June of 2020, PhRMA has convened thousands of stakeholders as we have worked to explore a new potential infrastructure with diverse communities, health systems and academia that seeks to show proof of concept for a network of connected, community-rooted trial sites.
With strong support from the biopharmaceutical industry, this effort seeks to create a sustainable, community-based infrastructure focused on clinical trial diversity
As we endeavor to push forward to change the future, we must first understand the past
Colonization, slavery, segregation, systemic racism—these experiences continue to disproportionately impact underserved communities and have created a foundation of mistrust rooted in history
Events like these lead to lasting mistrust and impact how communities of color approach health care. This mistrust, combined with social and economic barriers, is amplified when it comes to clinical trials. We are committed to working with communities to address this.
*This publication offers a limited number of free article views per month and then requires a subscription
Here is a small sampling of formative events in U.S. history that have shaped the relationship communities of color have with medical research and the health care system. This list is by no means exhaustive, but we hope it helps pave the way for candid dialogue that guides our work on equity.
Slavery
Gynecological Experimentation on Enslaved Women
Closing of Medical Schools and Exclusion of Future Health Providers
Birth Control Experimentation in Puerto Rico
The Tuskegee Syphilis Study
Henrietta Lacks
Radioactive Iodine
Slavery (1619 - 1865)
Gynecological Experimentation On Enslaved Women (1845 - 1849)

Closing Of Medical Schools and Exclusion Of Future Health Providers (1870)

Birth Control Experimentation in Puerto Rico (1930s - 1970s)

The Tuskegee Syphilis Study (1932 - 1972)

Henrietta Lacks (1951)

Radioactive iodine (1956 - 1957)
Resources

Equitable Breakthroughs in Medicine Development (EQBMED): 2022 Year-end Update
The EQBMED program, funded by a grant from the Pharmaceutical Research and Manufacturers of America (PhRMA), is an initiative to advance community-centric approaches to increase the participation of underrepresented patients in clinical research.
Stay Updated on PhRMA's Equity Initiative
Subscribe below for regular email updates as we push for necessary systemic and long-term change to improve health outcomes for underserved communities.